Project Grant 2016
Decomposition of pain into cell types
Principal investigator:
Patrik Ernfors, Professor of Tissue Biology
Institution:
Karolinska Institutet
Grant in SEK:
SEK 17.2 million over five years
“The sensory nervous system is really exciting. The hand alone has masses of sensory nerves in the skin that enable the brain to receive tactile information, i.e. feel whether a surface is smooth or rough, for example,” enthuses Ernfors as he strokes the surface of the table with his hand.
He is a professor of tissue biology at Karolinska Institutet, and is greatly interested in the mechanisms that control the formation and function of various nerve cells. He is leading a five-year project funded by the Knut and Alice Wallenberg Foundation that is centering on pain cells and their communication with each other. Ernfors and his research team hope to identify the exact cellular origin of pain in various pain states.
It is basic research, but research that may ultimately be of great benefit. Pain causes a great deal of suffering, with a high cost to society for health care and in working days lost. Around one in five people over the age of twenty experiences constant pain, and seven percent suffer debilitating pain, Ernfors explains. The drugs currently available, particularly those for chronic pain, are not good enough:
“They alleviate the pain, but they are not a cure, and they only work for half the people who take them. To create new, targeted drugs, we must understand what has gone wrong to cause pain. At present we don’t know the answer. We won’t be able to solve everything, but hopefully we will succeed in identifying some specific cell types and the part they play in pain.”
Answering questions with animal models
Sensory nerves are present in most organs and tissues in our body. When these nerves discover damage and inflammation they forward the information to the spinal cord, which codes it and sends signals to the brain. Chronic pain is caused by abnormal activity in sensory cells. Ernfors elaborates:
“We want to find out which pain cells become overactive, and how coding takes place in the spinal cord. Our hypothesis is that pain is caused by numerous different mechanisms. For instance, acute pain may originate in a certain type of neuron, whereas chronic pain is triggered in a different kind.”
Research in this field essentially trod water for three decades, but great strides have been made over the past few years. The impetus for research progress came from new methods enabling scientists to create genetically modified mice, known as “mouse models”, in which it is possible to turn cells on and off to understand their function.
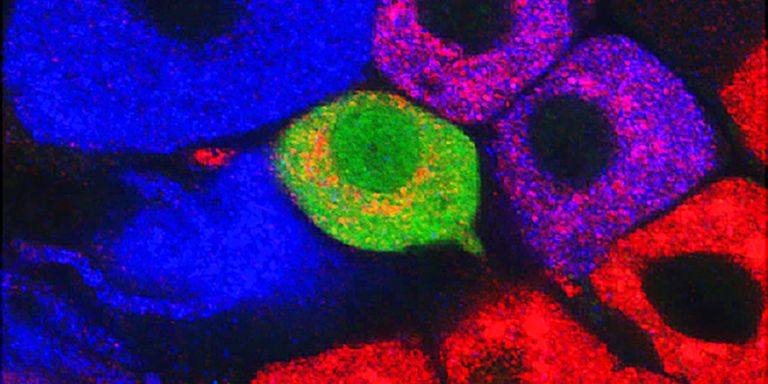
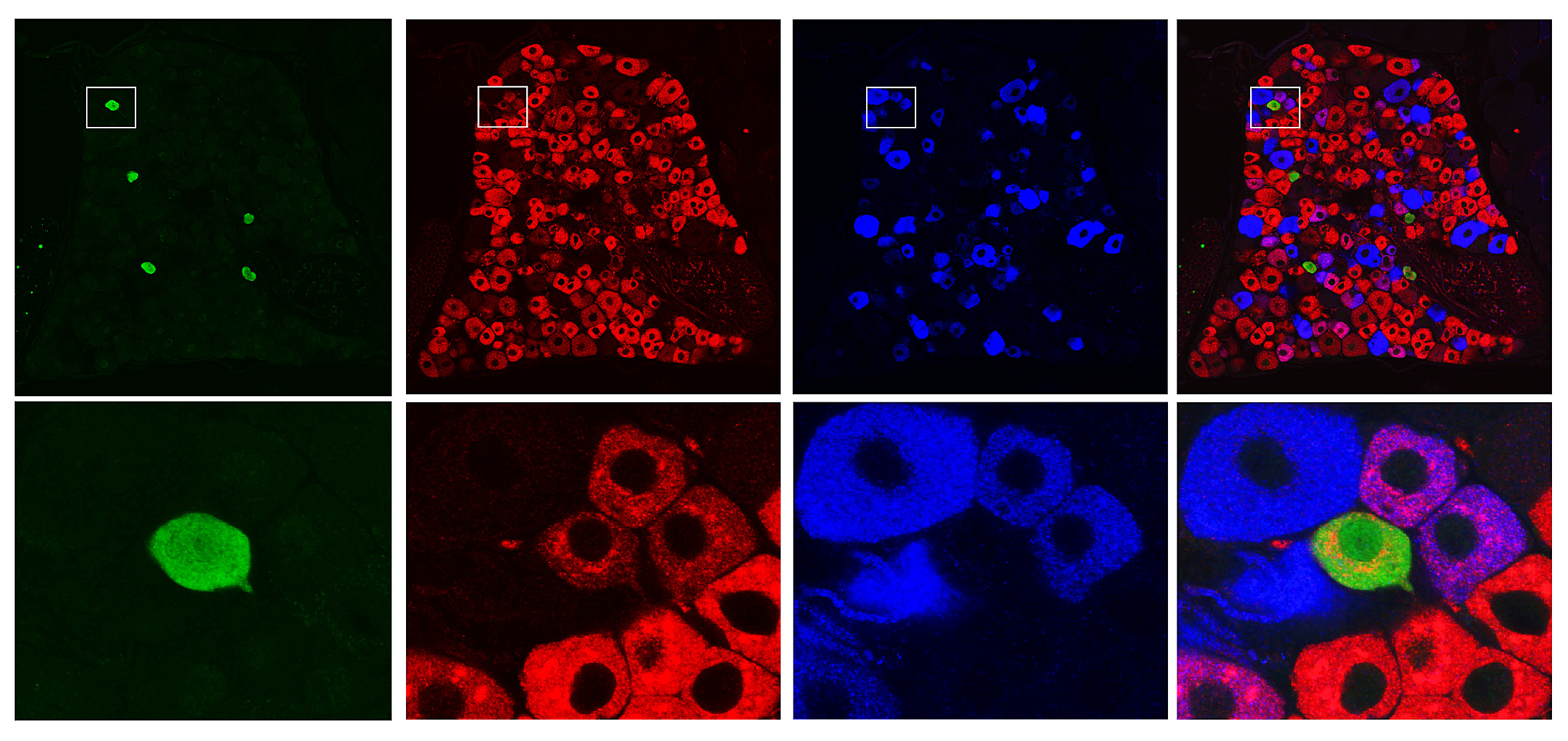
Ernfors’ team is using a number of advanced techniques in the lab. One of them is single-cell analysis, involving the study of individual cells separated out from living tissue. They study cells both in the dorsal root ganglion, i.e. sensory nerve cells throughout the body that send information to the spinal cord, and in the spinal cord itself.
“The next step will be to understand how they are connected to each other.”
Map of pain cells
Interesting data on pain cells in the sensory ganglion have already been produced. And the process of mapping the spinal cord is under way.
“It’s a herculean task. We have studied over 2,000 cells and found about 30 types that could be involved in pain. Around half of them turn off other neurons: the other half activate other neurons. Now we’re trying to make a physical map showing the anatomical location of the cells in the spinal cord.”
The project also entails sequencing – genetically mapping – macaques.
“If we find a cell type in mice that is critical for a given pain state, we can move on to our data on the macaque. If the same type of neuron is present in the ape, it is highly likely it will also be found in humans.”
The lab work generates copious quantities of data to manage and interpret.
“We can’t study all cell types. So it’s a great challenge to choose which ones we should focus on to find general principles on how they work. If we can understand the molecular identity of specific pain cells, it may lead to drugs that can inhibit the cells and stop the pain.”
New knowledge
In earlier studies Ernfors and his colleagues have identified a sensory cell that conveys touch sensation which can change function and send pain signals instead.
“When nerve pain occurs, this sensory nerve cell changes over from sending touch sensation to sending pain. This is incredibly interesting. It may explain what the textbooks call ‘lowering of the pain threshold’. From a molecular perspective, it is rather about a change in the function of a neuron.”
As mentioned, the technologies to makes studies of this kind did not exist even 5–10 years ago. Ernfors is elated over opportunities that have opened up.
“I feel that we will soon understand something that has been a mystery for a hundred years, which is what all researchers dream of.”
Text Susanne Rosén
Translation Maxwell Arding
Photo Magnus Bergström