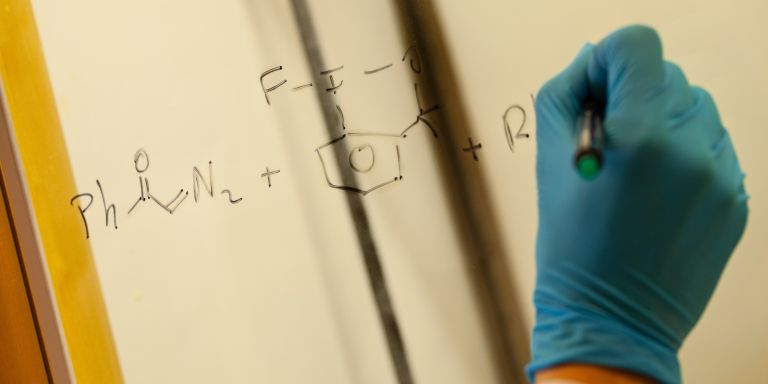Project Grant 2018
Organofluorines: anthropogenic small-molecules for life sciences
Principal investigator:
Kálmán J. Szabó, Professor of Organic Chemistry
Co-investigators:
Stockholm University
Pher G. Andersson
Fahmi Himo
Uppsala University
Eszter Borbas
Karolinska Institutet
Magnus Schou
Institution:
Stockholm University
Grant in SEK:
SEK 29.3 million over five years
“Designing and manufacturing new organofluorine compounds is a creative process – it’s a kind of craftsmanship. Everything we are doing here in the lab is unexplored territory,” says Professor Kálmán J. Szabó.
His research team is based at the Arrhenius Laboratory at Stockholm University. The researchers are making concerted efforts to develop new chemical methods of synthesizing specific organofluorines, i.e. molecules with at least one carbon-fluorine bond.
“Although fluorine is the 13th most abundant element in the Earth’s crust, there are only six naturally-occurring molecules with a carbon-fluorine bond. So these organofluorines are really the exception rather than the rule – it’s as though evolution has not wanted or been able to create them,” Szabó says.
But it is possible to make organofluorines artificially using advanced synthesis methods. Organofluorines have applications in the life sciences, and other fields. Twenty percent of all modern medicines are organofluorines, including the antidepressant Prozac, and Iressa, a cancer treatment drug.
“The carbon-fluorine bond is really strong. A drug is usually partly metabolized before it starts to act, which is naturally less than ideal, since this puts a strain on the body. In this sense, organofluorines are of interest, since they are not so easy to break down. They are also useful because their properties can be tailored to meet the needs of various drugs.”
Selective reactions
With funding from the Knut and Alice Wallenberg Foundation, Szabó’s research team is collaborating in a joint project with four other research teams from Stockholm University, Karolinska Institutet and Uppsala University. The project mainly involves basic research, although applications are also on their agenda.
Together, the scientists want to create new synthesis methods to tailor the complex organofluorines that play a key role in development of new drugs, and in clinical diagnostics using PET (Positron Emission Tomography). The project includes synthesis of safe, stable reagents for fluorination, and use of catalytic methods.
Szabó elaborates:
“We’re using catalysts that speed up the reactions, but most of all, that make them more selective. Selectivity is a key word, as it is a question of synthesizing molecules for medicinal and diagnostic use. Ultimately, we need a single substance with a very high degree of purity.”
PET and fluorine-18
PET is used in medicine for early diagnosis of neurological diseases such as Parkinson’s and Alzheimer’s. The contrast substance injected in patients uses the radioactive isotope fluorine-18 to label radioactive trace molecules. For instance, a molecule may selectively bind to misfolded proteins formed in Alzheimer’s disease. The isotope emits positrons, which are annihilated and the generated gamma rays are recorded by the PET camera.
Szabó explains that PET is an incredibly powerful method, but the challenge is that each disease requires specific molecules labeled with fluorine-18:
“Our work is synthesizing new fluorine-18-labeled radiotracers for PET in order to study biochemical processes in the body. We are collaborating with the research team at Karolinska Institutet in our efforts to find completely new methods for fluorine-18 labelling of PET tracers. We have no shortage of ideas, so we will have to see what we are able to come up with.”
Since fluorine-18 does not occur naturally, a PET examination only requires minute quantities of tracer molecules
“Nanogram quantities of labeled molecules suffice to obtain a clear picture of the extent of the disease. This is great for patients, who are not harmed by the examination. But it also represents a huge challenge for chemists like us who have to work with substances in such infinitesimally small quantities,” Szabó says.
Pre-eminent fluorine research
One reason that organofluorines are so difficult to create, both for Nature and for organic chemists, is that fluoride ions have properties that make them very unreactive. Szabó explains:
“It’s sometimes said that fluorine is a small atom with a big ego. Organofluorine chemistry demands innovative thinking and novel methods of organic synthesis. Complex reaction mechanisms have to work together to achieve the desired reactivity and selectivity. To this end, use quantum chemical computer modeling, to try to explain the observations in the lab, and also to make predictions about what methods we should apply.”
One of the major goals of the project is establishment of pre-eminent fluorine research in organic chemistry in Sweden. Szabó sums up:
“Currently, there is not so much other research in organofluorine chemistry in elsewhere in Sweden. We spent a long time pondering the very long duration of the project, which requires collaboration by multiple research teams possessing a variety of skills. The grant has now enabled us to carry out the project.”
Text Susanne Rosén
Translation Maxwell Arding
Photo Magnus Bergström