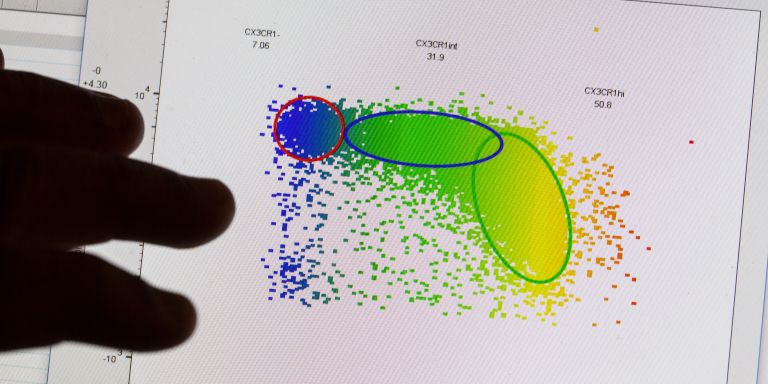
Human biology has always fascinated Carmen Gerlach. Having been chosen as a Wallenberg Academy Fellow, she is exploring the workings of our immune system. If we can learn from nature’s own mechanisms, it may be possible to develop better vaccines and more effective immune therapies for cancer and various autoimmune diseases.
Carmen Gerlach
Doctor of Medicine
Wallenberg Academy Fellow, Prolongation grant 2024
Institution:
Karolinska Institutet
Research field:
Fundamental principles and mechanisms of T-cell immune reaction, with particular emphasis on CD8-T-cells.
Irrepressible curiosity drives Gerlach to devote her energies to basic research in the field of biomedicine. She is inspired by nature’s evolutionary achievements.
“The human body is an incredible piece of engineering that nature has evolved and refined over the millennia. And there is much we can learn by investigating what evolution has created.”
Even as a young student back in the city of Leiden in the Netherlands she wanted to understand how the human body works. For a while she was drawn to neuroscience, but in the end she chose to specialize in immunology.
“Sooner or later you have to specialize in order to be good enough. Research is my passion, and part of my identity, just as sport is for some people.”
Moving to Sweden
Gerlach has now transferred her research from Harvard Medical School to Karolinska Institutet, an environment she got to know back in the days when she was a young exchange student. She is continuing her work on unraveling the immune system. It is an ingenious system, part innate and part adaptive, the latter enabling it to learn and become stronger. The innate elements are the first line of defense – capable of reacting swiftly to infection. The adaptive immune system often reacts more slowly, but can instead provide life-long protection.
The adaptive, or acquired, immune system is controlled by lymphocytes – white blood cells that specialize in combating viruses and bacteria. Lymphocytes get activated when foreign antigens enter the body. They help to resolve the infection, for example by producing antibodies, or by killing the infected cells in the body.
“What I wonder is how this system can respond so effectively to so many different kinds of attack – from virus infections to a tumor, parasite or bacterium.”
“Long-term funding of this kind is central to the research climate in a country. I am driven by a desire to conduct research that makes real strides, although scientific breakthroughs do take a long time. It is fantastic that the Foundation wants to invest in research that involves a high risk, but that also offers the potential to yield great rewards in the future.”
Nobel Prize for breakthrough
Knowledge about the immune system has been used to develop modern-day vaccines, which work by triggering cells to produce antibodies. Another field concerns immunotherapy for cancer. In 2018 the Nobel Prize in Medicine was awarded for discoveries establishing a new principle for cancer treatment by inhibiting the natural “brakes” in the immune system.
“Unfortunately, there are still diseases for which there are no effective vaccines. And we know that the body’s immune system does not cope optimally with tumors. Hopefully, if we can learn more about why the immune system is effective in one situation, we will be able to transfer that knowledge to other contexts.”
The research requires in-depth studies of the various components of the immune system, such as T-lymphocytes. They mature behind the breastbone in the thymus, and possess many useful characteristics.
Gerlach is concentrating on a central group called CD8-T-cells. Some of them act as armed forces – eradicating infected cells in the body so that infection does not spread. Others form long-lived memory cells that are able to remember microbes that have invaded the body. If a virus or a bacterium makes a new attempt to enter the body, the memory cells recognize the infection, and are ready to battle, often even before the infected person feels ill. Meanwhile, the memory cells produce more memory cells to protect against future infections.
“There are numerous kinds of CD8-T-cells. All of them are actually individuals, just like people, but it’s possible to identify different characteristics and group them accordingly. It’s a bit like different occupations, such as bakers or plumbers.”
Part of the research is now focusing on identifying and analyzing the behavior of the various cells in detail. Among other things, Gerlach wants to understand how important the absence or presence of certain groups of cells is to the immune system.
“If we continue the occupational metaphor: if there are too few plumbers for all the work to be done, society must take steps to encourage more people to train as plumbers. There has to be a balance between supply and demand. It’s the same with the immune system. If we want to develop a better vaccine, we may have to encourage the younger generation, that is, cells of a certain kind, to train for the right kind of job.”
But there may also be other problems. Plumbers depend on their hands, but a tumor may have fettered the “hands” of the cell so it cannot do its job.
“The Nobel Prize was about loosening the rope around the hands so that the plumber can start to work again. We have to understand that there are numerous processes operating at different levels the whole time.”
Mapping immune cells
Gerlach is using a number of techniques to map the origin and identity of the cells. These include flow cytometry and single-cell RNA sequencing. Much remains unknown about how cells acquire their different characteristics, but greater knowledge may even enable scientists to induce specific, desirable cells.
“The result may be better vaccines or therapies for autoimmune diseases, as well as more effective cancer therapies. But what drives me is not the idea of curing a given disease – I want to do as much as possible to understand the underlying biology in the hope that this will lead to major medical breakthroughs in the future.”
Text Nils Johan Tjärnlund
Translation Maxwell Arding
Photo Magnus Bergström