Project Grant 2018
Capturing the regenerative potential of the brain
Principal investigator:
Malin Parmar, Professor of Cellular Neuroscience
Co-investigators:
Lund University
Tomas Björklund
Karolinska Institutet
Thomas Perlmann
András Simon
Institution:
Lund University
Grant:
SEK 21.6 million over five years
People suffering from Parkinson’s lose brain cells that produce dopamine, a signal substance affecting everything from movements and blood pressure to speech, sleep and mood. The motor symptoms in Parkinson’s disease can be alleviated with drugs or electric implants in the brain. Dopamine cell transplants have also been tested – using cells taken from aborted fetuses. This can work very well, but use of fetal cells raises ethical issues, and their availability is very limited. It would be much better if our brains could regenerate their own dopamine cells, or if other cells in the brain could change identity.
“Here in Lund we were the first in the world to show that it’s possible to reprogram a human skin cell to become a dopamine-producing neuron without the intermediate stem cell stage. We then concluded that it ought to be possible to this in situ, in the brain, if the right genes can be inserted into the right cells,” says Malin Parmar.
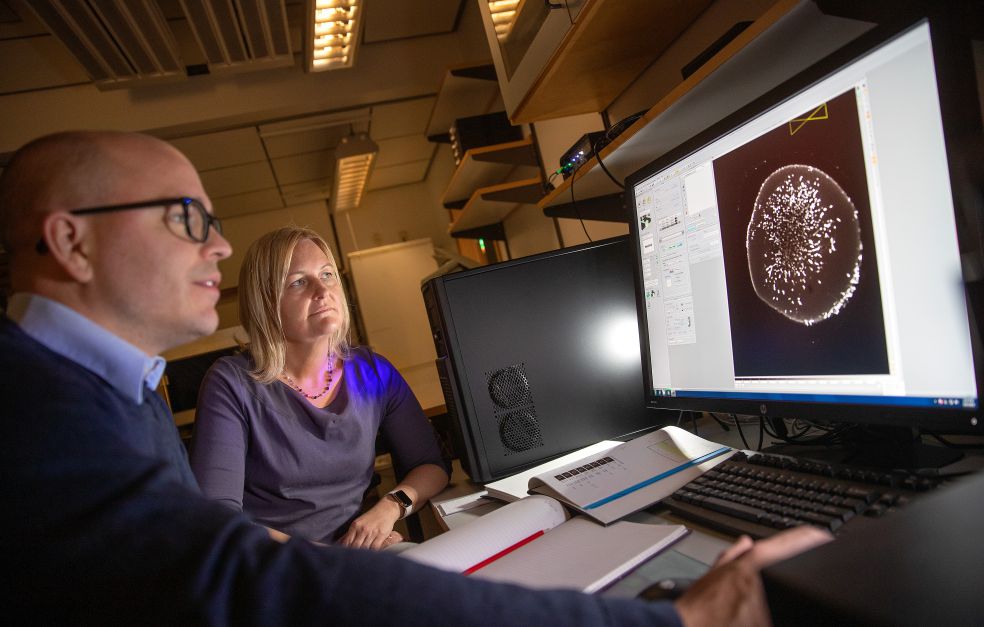
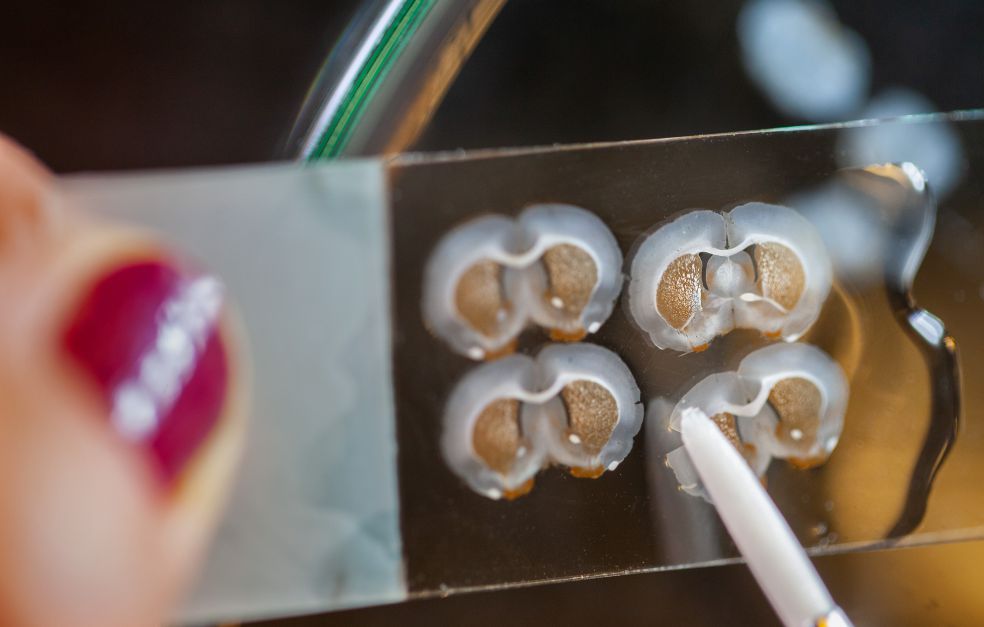
She is Professor of Cellular Neuroscience at Lund University, and heads a project funded by Knut and Alice Wallenberg Foundation that is studying the possibility. The project team is studying salamanders, which are able to repair brain damage naturally. The researchers hope to be able to use the genes activated when the salamander is harmed as “reprogramming genes” for also creating new cells in the rodent and human brain
Giving the genes a good reception
“What we’re doing lies on the borders between medicine, technology and mathematics,” says Tomas Björklund, a researcher in molecular neuromodulation at Lund University.
He is one of the research team leaders in the project, which combines several fields of knowledge: gene therapy, new cell formation, single-cell sequencing (which maps all active genes in individual cells), and artificial intelligence. The researchers are using machine learning to analyze the entire system surrounding a dopamine cell, from formation to the place occupied by the cell in an advanced communication system.
“Dopamine neurons are involved in everything from motor skills to the brain’s reward system. Their function is completely dependent on the other brain cells with which they communicate, and on which genes are active. We will be trying to introduce the genes we map in salamanders into human cells, to see if they can recreate functioning dopamine cells,” Björklund says.
But the human cells are not being studied in humans. Instead, the researchers are using rats that have been modified to have human-type dopamine-producing cells. Earlier research shows that mice have genes similar to those of salamanders – genes that might be able to achieve the same thing if scientists knew how to activate them.
But it is not enough for the researchers to succeed in reprogramming other brain cells, i.e. “support cells”, known as glial cells, so they form dopamine-producing cells. They also have to interact in exactly the right way with the cells already present in the brain.
“We will be studying what happens to a new cell in the old brain. How do the cells already in place teach the new cell what to do? This is relevant to many diseases, not just Parkinson’s,” Parmar says.
Basic research with an eye on medical treatment
Tomas Björklund also stresses the importance of the connections between neurons and other cells. Malin Parmar and he have both been involved in previous research projects in which they have studied how cells in an ageing brain can also create synapses – connections – with newly-formed dopamine cells. The new dopamine cells also create synapses to cells in other areas of the brain.
“This is a fairly recent – and astonishing – discovery. It means we have to find out which of the older cells communicate with the newly-formed dopamine cells. Is it a help or a hindrance? Can we control it so that the brain regenerates exactly the right circuits? If the result is new neurons with the wrong synapses, problems may become worse rather than better,” Björklund comments.
The project largely involves basic research – learning more about genetics, how cells can be controlled using genes and chemical signals, and what is needed to ensure that a cell works well. The aim is to find more sophisticated methods of controlling cells, and gaining a better understanding of Parkinson’s disease. Studying gene activity in dopamine cells may offer a path to new discoveries about the genes that play a part in triggering the disease.
Although the project is not intended to yield a ready-to-use medical treatment, the researchers have chosen to use tools and methods that are already included in gene therapies used in health care. One example is virus capsules genetically modified to serve as harmless vectors for the genes intended to be inserted in the body.
“In five years’ time I hope we will have shown in our animal models that this method of creating dopamine cells works just as well as cell transplants. Best of all would be if we could inject the genetically modified viruses directly into the bloodstream. That’s where the threshold to clinical trials is lowest,” says Parmar.
Text Lisa Kirsebom
Translation Maxwell Arding
Photo Magnus Bergström