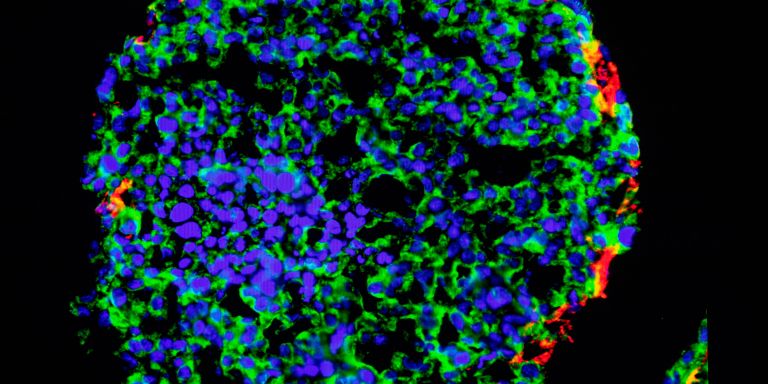
The obesity epidemic sweeping the world means that growing numbers of people suffer from fatty liver – a potentially fatal disease for which there is no effective treatment. Wallenberg Academy Fellow Stefano Romeo wants to change this. He is seeking explanations and targets for new drugs by comparing the DNA of healthy people with that of fatty liver sufferers.
Stefano Romeo
Professor of Molecular and Clinical Medicine
Wallenberg Academy Fellow 2017
Institution:
University of Gothenburg
Research field:
The impact of genetic variations on blood lipids and metabolic liver disease
Fatty liver, which can lead to cirrhosis and cancer, may arise due to excess consumption of alcohol. But it is also a common consequence of overweight and obesity. The obesity epidemic we see today has meant that fatty liver is now one of the commonest diseases in the developed world. About one-third of all adults are affected, and as yet there is no effective treatment for the condition. In serious cases the only cure is a liver transplant.
“Lifestyle changes are also effective, but research has shown that this approach is seldom sustainable. Patients make the necessary changes, but then revert to their former lifestyle. It is exactly the same problem as with obesity. After all, fatty liver is really a phase of obesity,” Romeo explains.
Romeo is a physician and professor at the Department of Molecular and Clinical Medicine at the University of Gothenburg. He spends about one-third of his time working at Sahlgrenska University Hospital, where he treats patients with elevated cholesterol. The rest of his time is spent at the Wallenberg Laboratory at Sahlgrenska researching into the genetics of fatty liver. His aim is to lay the foundations for new drugs.
“We are studying the genes of individuals who do not develop fatty liver even though they fall in the high-risk category, to identify gene variants that protect against the disease. On that basis we can draw inferences about the mechanisms that control fatty liver, and try to find ways of controlling them,” Romeo says.
His research team is using large quantities of genetic data from healthy people and patients with fatty liver to search for gene variants that appear significant, either because they cause disease, or because they protect against it. Those genes can then be inserted in cells in the laboratory, enabling the researchers to study their activity in the cell, and test whether it can be controlled with drugs.
All genes in a cell code for proteins produced by that cell. When the researchers know which proteins appear to be linked to a disease, and which ones inhibit it, they can look for ways of inhibiting or promoting synthesis of those specific proteins.
“We have found a protein with a variant that causes disease by impacting multiple cell types in the liver. We have genetically modified mice with fatty liver, so they produce less of that protein, and this had a clear impact on the disease,” Romeo explains.
Bonus effect on diabetes
There are already cancer care therapies based on suppressing production of proteins with specific changes, known to drive the disease. Romeo hopes a drug of this kind can also be developed for fatty liver.
“Modern gene technology offers good tools for blocking or reducing the synthesis of specific proteins in the cell. Also, large genetic information databases are still being analyzed, both by our team and by other researchers. This will enable us to discover even more potential targets for drugs.”
It is likely that any such drug will also affect the metabolism of glucose in the body, thereby impacting type 2 diabetes. But although Romeo has made considerable progress, many challenges lie ahead. He stresses the importance of working with other researchers, and openly discussing the data obtained. Data must always be interpreted – the trick is to find the best and most accurate interpretation.
“I actually think the greatest challenge is to focus on the goal. You can’t fall in love with a hypothesis – you have to let the data speak for themselves. So far, fortunately, the data would suggest that our hypothesis is correct.”
Doctor at a higher level
Even as a child, Romeo wanted to be a doctor. Having completed his medical studies in Rome, he moved to Dallas, Texas, where he spent a number of years studying for his PhD. It was there that he decided he wanted to make a long-term commitment to research. He was fascinated by the passion he saw in his former mentors in the US, which he found infectious.
“Research is like going places where no one else has been – or examining clues to find the murderer. It’s exciting and loads of fun. Research is the most enjoyable part of my life.”
“Being chosen as a Wallenberg Academy Fellow was unexpected, and a tremendous honor. The best thing about the award is that it puts me in touch with top researchers in other fields whom I would never have met otherwise. We have different interests, but the same passion for what we do.”
But Romeo also enjoys clinical work. Essentially, he feels like a doctor even when he is conducting research.
“After all, if you do in fact manage to discover a drug, it means you’ll be able to treat far more people than you could as a doctor. Research is like being a doctor at the very highest level – so I’m still doing the job that I originally wanted to do.”
Text Lisa Kirsebom
Translation Maxwell Arding
Photo Magnus Bergström