“When we understand how it works, we will be able to block the transmission of parasites between people and mosquitoes” he says.
“When we understand how it works, we will be able to block the transmission of parasites between people and mosquitoes” he says.

Project Grant 2018
The systematic identification of parasite gene functio
Principal investigator:
Professor Oliver Billker
Institution:
Umeå University
Grant in SEK:
SEK 30 million over six years
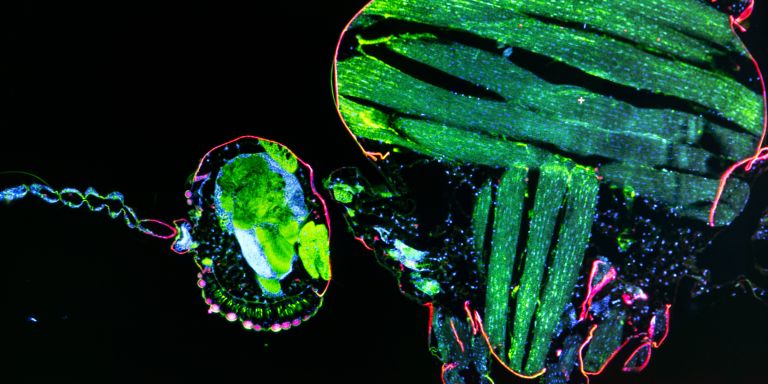
Every year malaria kills more than 400,000 people, most of them children. The disease is spread by mosquitoes that carry Plasmodium, a unicellular parasite.
“The parasite needs both a host organism and a mosquito vector to survive,” Billker comments.
Parasite spreading disease
The Plasmodium parasite moves between individual organisms in a well-orchestrated cycle. When a previously uninfected mosquito sucks the blood of a person with malaria, it takes up specialized male and female parasites, whose only role is to infect. They unite and form a motile cell that can penetrate the mosquito’s intestines. The cell grows and eventually releases thousands of small parasites, known as sporozoites, which spread to the mosquito’s saliva glands. The sporozoites will be transmitted to the next individual the mosquito bites, where they first take up residence and grow in the liver, before the red blood cells get infected.
The parasite thus gets transmitted between mosquitoes and humans, multiplying as it does so. Only a few parasites manage to move between hosts, which makes them vulnerable to interventions that block transmission.
“We want to understand the parasite’s behavior. If we can find out how it moves between mosquitoes and humans, we can look for ways to prevent that from happening.”
Studying malaria in mice, Billker’s research team examines both the parasite’s genes and its life cycle. The project involves switching off each of the parasite’s several thousand genes in turn, and studying the effect on the parasite to work out what each gene does.
“The ability of malaria parasites to move between different types of organisms while also multiplying rapidly has always fascinated me,” Billker remarks.
Previously, working with his colleagues, he has developed new methods of using a single experiment to determine how thousands of parasite genes function in individual cells. They are now using the same techniques to map the genes that regulate the complex life cycle of the malaria parasite.
“We know that the parasite has a fairly small genome, with genes that are highly specialized for their purpose. We’re studying them not one by one, but all of them in parallel.”
Major international collaboration
Billker studied biology in Berlin and received a PhD from the University of London. After a couple of years at the Max Planck Institute for Infection Biology in Berlin, he returned to London to set up a research team at Imperial College. In 2007 he moved to the Wellcome Sanger Institute in Cambridge, one of the world’s leading institutes for genome research.
“I had a fantastic team in Cambridge”, he recalls, “and the new genetic tools we managed to develop have greatly improved the rate at which we can now understand how malaria parasites work”.
Two of his former colleagues at Cambridge accompanied him in 2018 when he was appointed Director of the Laboratory for Molecular Infection Medicine (MIMS) at Umeå University. He also brought with him eggs from a malaria mosquito that had originally come from India. Since then, MIMS has also imported African mosquitoes.
“Naturally, there is a risk that some of our observations with laboratory models cannot be extrapolated to human malaria. We therefore collaborate with fellow researchers in tropical countries, who can compare our findings with their own field studies. They help us to ensure that our ideas on transmission between mosquitoes and humans are in fact correct.”
In the fall of 2019 Billker and some 20 of his colleagues from four countries presented their findings from a systematic analysis of malaria parasite genes throughout its life cycle. They carried out a large-scale experiment in which they knocked out 1,300 individual genes and found 461 of them are needed so the parasite can be transmitted to mosquitoes and back to the host animal’s liver and bloodstream. Their next step was to create a model of the parasite’s metabolism.
“We have shown how the parasite’s metabolism changes to rapidly multiply in the host animal’s liver. We believe this has revealed new ways of targeting this life cycle stage with much-needed drugs.”
They now collaborate with a pharmaceutical company keen to find new compounds that can kill the liver stage.
Umeå – a flourishing research environment
Billker is convinced that Umeå offers an outstanding research environment because it combines cutting edge infrastructure with a great collaborative spirit. It is also home to a wealth of knowledge about genetic tools.
A six-year research grant from Knut and Alice Wallenberg Foundation provides the team with the stable funding they need to tackle big scientific questions.
“When offered the position as Director of MIMS, I didn’t have to think twice before accepting because I knew our research could continue unabated, thanks to the long-term funding the Foundation made available. Knut and Alice Wallenberg Foundation really stands out among Swedish funders when it comes to providing support at a scale that stimulates new ideas.”
Text Carin Mannberg-Zackari
Translation Maxwell Arding
Photo Magnus Bergström
Facts
Malaria mosquitoes make up a genus containing about 400 species. About 40 of them spread Plasmodium parasites, which cause malaria, an infectious disease.
Malaria mosquitoes are found on all continents except Antarctica, but are most common in tropical regions.
Nearly half the world’s population runs the risk of malarial infection. Most people have insufficient access to protection or treatment.
There is still no effective vaccine. Sweden also has several species of malaria mosquito. They are not infected with the malaria parasite, however.
MIMS and EMBL
MIMS is a partner institute of the European Molecular Biology Laboratory (EMBL) in Heidelberg, together with a network of other Nordic research centers.
The network offers scientific infrastructure and research expertise in molecular medicine aimed at understanding diseases and finding new treatment methods.
More about Oliver Billker's research
Groundbreaking malaria research could save millions of lives