Project Grant 2016
Molecular X-Ray Micro Imaging
Principal investigator:
Hans Hertz, Professor of Biomedical Physics
Co-investigators:
KTH Royal Institute of Technology
Muhammet Toprak
Karolinska Institutet
Marie Arsenian Henriksson
Institution:
KTH Royal Institute of Technology
Grant in SEK:
SEK 33,090,000 over five years
There is a huge demand in biomedical research and the health care sector for new imaging methods capable of producing more detailed images. A number of techniques are used at present, but they all have limitations. X-ray technology provides the best resolution, down to 0.5 mm in humans and sometimes 0.1 mm in animal models. But the information provided by X-ray images is virtually all structural, whereas it is becoming increasingly important to also obtain images of molecular function to understand the progress of various diseases. Hans Hertz, Professor of Biomedical Physics at KTH, explains:
“X-rays show us mainly bones. MRI – magnetic resonance imaging – enables us to see some function, and PET – positron emission tomography – allows us to study both molecular processes and function, albeit at the expense of resolution, 1–2 millimeters. What we are now looking for is the Holy Grail – a combination of high spatial resolution and function.”
The aim of the new project is to image molecular function down to 0.1 mm, i.e. ten times more sharply than is currently possible. Hertz draws a comparison:
“Early detection of tumors is essential if we are to deal with them before they have had time to spread. Being able to detect tumors when they are ten times smaller is even more important. We hope realistically to achieve this in pre-clinical trials on small animals.”
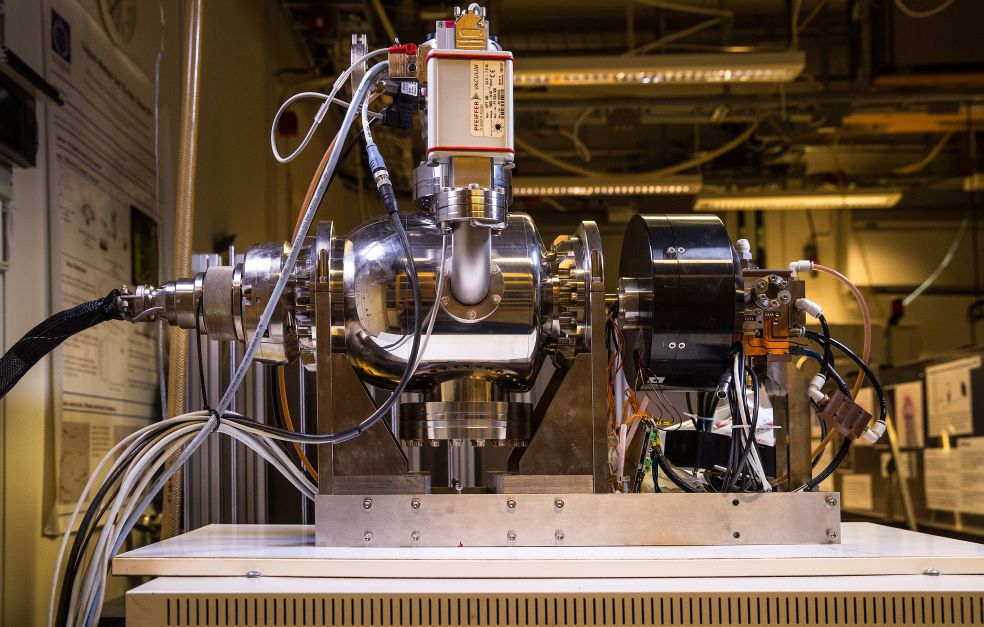
Innovative X-ray source paves the way
The method is based on a refinement of X-ray technology that Hertz and his colleagues at KTH began to develop as far back as the 1990s. The new X-ray source has attracted a great deal of attention, and is seen as the most important breakthrough in the field since the 1930s. The source generates more photons per second and area than older X-ray tubes. This increases light intensity, and paves the way for new applications.
“Our source creates stronger light than other X-ray sources, and is essential to this project. But we are also using it for other projects.”
The innovation is called liquid metal jet technology. Simply put, the researchers have replaced the fixed metal anode in the X-ray tube with a jet of liquid metal. This makes it possible to increase the power of the electron beam in the X-ray apparatus without any risk of the anode overheating or melting, since it is already in a liquid state. The higher intensity in the X-ray tube allows shorter exposure times, which is also needed if the technology is to be feasible for practical use.
Wallenberg Foundation funding essential
As a Wallenberg Scholar, Hertz has continued to research into potential applications, and his new research project is also being funded by the Knut and Alice Wallenberg Foundation.
“The grant we have received from the Wallenberg Foundation is absolutely essential in order to build the multidisciplinary infrastructure needed, and to achieve something out of the ordinary, involving everything from advanced X-ray physics to experiments using small animals and advanced nanoparticle chemistry.”
The immediate objective is to develop the method for use on animal models in pre-clinical research. The research team is working closely with fellow researchers at Karolinska Institutet on a facility being built at KTH, where the new X-ray imaging method will be tested.
Tailored nanoparticles searching for tumors
The researchers have enlisted the help of X-ray fluorescence from metallic nanoparticles tailored for the purpose. Targeted nanoparticles are a rapidly growing research field. These nanoparticles can be given specific surface molecules that bind to tumor markers.
Hertz elaborates:
“We make our own nanoparticles and test their function. After we have injected them into the animal models, we wait a day or so while the particles gather in a tumor, for example, before taking an X-ray to see where they are.”
The search is made easier by the fact that blood vessels grow more quickly in and around a tumor, and when they burst, the nanoparticles seep out to a greater extent. The nanoparticles are made from molybdenum, an element whose properties match the X-ray source well. A special team of three or four researchers is now working on nanoparticle production, which is a central part of the project.
If the imaging method works in animal models, Hertz hopes to go on to clinical use of the method on humans. But he stresses that it is still early days:
“As things are at the moment, it looks like we really will be able to increase spatial resolution tenfold in mice. But humans represent a much greater challenge, and there are numerous complications. But even in the mouse models the method may have an impact on research into tumor biology, and on development of new drugs.”
Text Nils Johan Tjärnlund
Translation Maxwell Arding
Photo Magnus Bergström