Diseases like Alzheimer’s and Parkinson’s are primarily associated with protein aggregation, but also create a kind of traffic jam in the brain’s neurons. Elin Esbjörner wants to improve our understanding of how this blockage forms. This may pave the way for new treatment strategies.
Elin Esbjörner
Associate Professor of Chemical Biology
Wallenberg Academy Fellow, Prolongation grant 2024
Institution:
Chalmers University of Technology
Research field:
Protein aggregation mechanisms and amyloid formation in neurodegenerative diseases
A common feature of neurodegenerative diseases such as Alzheimer’s, Parkinson’s, amyotrophic lateral sclerosis (ALS) and Huntington’s is the formation of clumps of misfolded proteins in the brain. These aggregations fasten around or inside neurons, which find it increasingly difficult to communicate with each other.
Much of the current research on Alzheimer’s and Parkinson’s aims to find methods of getting rid of these plaques or preventing them from forming.
“The strategy is probably sound, but we still don’t have a method that works, so we also need to explore other avenues. I hope I’ll be able to add some pieces to the puzzle,” says Esbjörner.
The cell as a city
She likens cell transport systems to urban traffic systems. Multiple systems are needed for the city to function: from courier vehicles and buses to garbage trucks. Our cells have similar traffic systems to receive the various substances needed in the cell, and to dispose of waste, including waste proteins.
But why do cells fail to remove protein aggregations in neurodegenerative disease? Esbjörner wants to identify the weakest link in the chain.
“We want to understand what happens – is it damage to the transport system that causes the protein aggregates to accumulate or is it the protein aggregates that clog up the system?” she muses.
She and her research team intend to find the answer by developing tools to monitor the “vehicles” – endosomes – responsible for the transport itself.
“Endosomes have different identities, and make their way to different parts of the cell. They can be likened to different city bus routes. We’re developing methods to label them so we can see the route they take inside the cell, and measure what they contain,” she says.
Tailored proteins
First they tailor the protein aggregates themselves in laboratory test tubes. This is to see whether different protein traits affect their behavior inside the cells. It also enables the team to study how biological and pharmacological substances impact the proteins. The aggregates are then presented to cells, to be gathered up by the labeled endosomes.
“This enables us to monitor the impact of different protein aggregates on the transport systems. But it’s not easy to do this in living cells, not least because endosomes are so small and numerous, and move quite quickly,” she explains.
For this reason, the team will also be developing methods of extracting endosomes from cells to analyze them one by one.
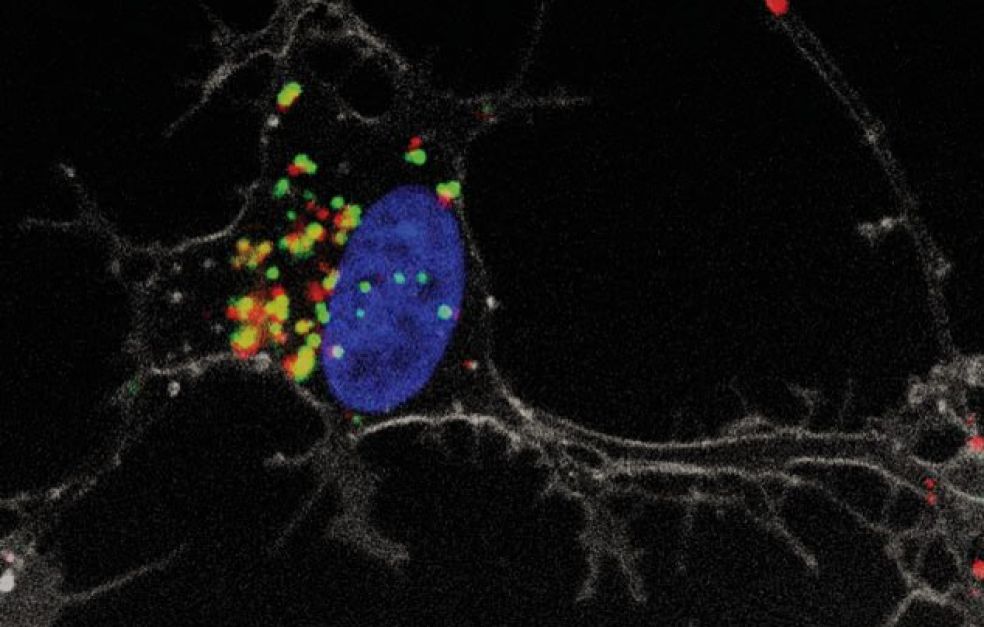
Fluorescence is one of the techniques they are using. They have identified molecules that fluoresce differently, depending on whether or not protein aggregates form. This makes it possible to monitor protein aggregation using advanced microscopy methods.
Fascinating by the structures
Esbjörner’s interest in protein aggregation was awakened when she saw microscope images of misfolded proteins.
“Protein aggregates are long threads twisted into a spiral form – very attractive structures. They are arranged in tiny tangled matrices, and I was quite simply fascinated by the structures, and wanted to understand them better,” she says.
She also mentions a more personal motive for wanting to gain a better understanding of neurodegenerative diseases.
“One of my aunts lived for many years with Parkinson’s disease, so I personally feel this is a really important area in which I can make a contribution.”
After three years as a postdoc in Cambridge, England, she returned to Chalmers University of Technology in Gothenburg to set up her own research team.
“After my time at Cambridge, I knew I wanted to explore the crosstalk between the toxic aggregates and the transport systems, since they are so central to neural function. If we can find a way to prevent the aggregates accumulating, or correct transport systems that are not working, it may offer an alternative approach to curing neurodegenerative diseases,” Esbjörner says.
“Being chosen as a Wallenberg Academy Fellow provides financial security, which makes it easier to adopt a more long-term approach.”
Paving the way for genetic therapeutics
The basic knowledge being established by the research team may also have a broader application, not least in understanding cellular uptake of the tailored aggregates. Cellular uptake has been a specialty of Esbjörner’s ever since she received her PhD.
She contributes her expertise to the FoRmulaEx research center, where better methods of delivering genetic drugs to cells in the body are being developed. Examples of such therapeutics include the first vaccines for Covid-19.
“There is a pressing need to solve the problem of transport into the cells. The large mRNA molecule has to be encased in special packaging in order for cell uptake to occur. If we can contribute further basic knowledge, we will be able to accelerate the development of new vaccines,” she says.
The methodological strides made at Chalmers are one of the reasons she has remained faithful to her alma mater.
“I am driven by the desire to solve a biomedical problem, but there are plenty of other researchers here who are driven by the challenge of developing new methods. This creates fruitful synergies, enabling us to make more progress together.”
Text Magnus Trogen Pahlén
Translation Maxwell Arding
Photo Daniel van Leeuwen, Mikael Winters, Marcus Marcetic